Near-Miss Epidural Administration of Tranexamic acid (TXA)
This content has been developed for SALG by the consultant anaesthetist involved in the individual case.
A patient underwent caesarean birth under epidural (‘top-up’) anaesthesia. Shortly after delivery, the patient started to haemorrhage and 1g tranexamic acid (TXA) was requested. While preparing the drug for administration, one of the ampoules of TXA was mistakenly replaced with an ampoule of preservative-free morphine, intended for administration via the NRFitTM epidural catheter. The error was recognised when the ampoule of TXA was seen in the colour-coded epidural drug tray before the epidural drugs had been drawn up, but after the woman had been given the morphine intravenously.
Commentary
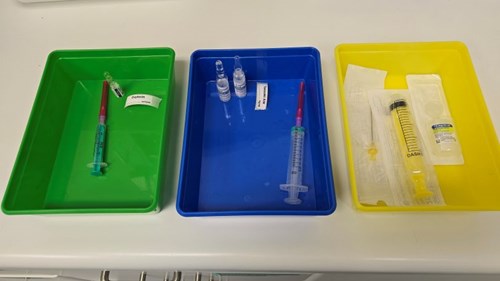
In 2022, the WHO published a Medical Product Alert1 warning of the serious risks associated with intrathecal administration of TXA, including a mortality rate of 50%. There has been at least one case report of inadvertent epidural administration of TXA2, which appeared to cause less harm than the intrathecal route (possibly due to intact dural protection and use of saline lavage). Use of NRFitTM connectors for neuraxial drug administration reduces the risk of wrong route error3 but does not eliminate it, as there is no protection when the wrong drug is drawn up into the neuraxial syringe. Other safety systems, such as two-person checks, can be (and are) ‘worked around’ during high acuity situations. The use of a specified colour-coded tray for epidural equipment and drugs may well help to identify substitutions errors like these (see photo), although there is nothing to stop the syringe/ ampoule from being placed in the wrong tray
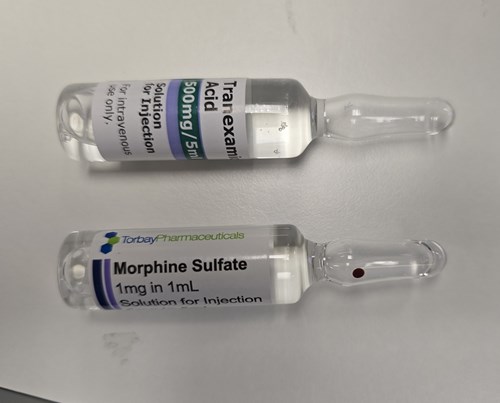
Encouraging vigilance around ‘slip errors’ (i.e. an error that occurs due to a lapse in attention) is largely ineffective. Practical measures, such as designating TXA a ‘high risk’ medication to be stored away from other drugs, are more effective. Manufacturers are encouraged to label ampoules to highlight the contents (rather than the manufacturer’s branding) to reduce the risk of inadvertent substitution (see photo).
Pre-filled Luer syringes of TXA would significantly reduce the risk of accidental neuraxial administration. Unfortunately, these are not currently available. SALG will be asking manufacturers of pre-filled syringes to prioritise this product for development.
- World Health Organization. Risk of medication errors with tranexamic acid injection resulting in inadvertent intrathecal injection.March, 2022 [Accessed 26th February 2025]
- Pysyk, C.L. and Filteau, L. Accidental administration of tranexamic acid into the epidural space: a case report. CJA 2022 Jun, 69(9): 1169–1173.
- NHS England. National Patient Safety Alert – Transition to NRFitTM connectors for intrathecal and epidural procedures, and delivery of regional blocks. January, 2024 [Accessed 26th February 2025]